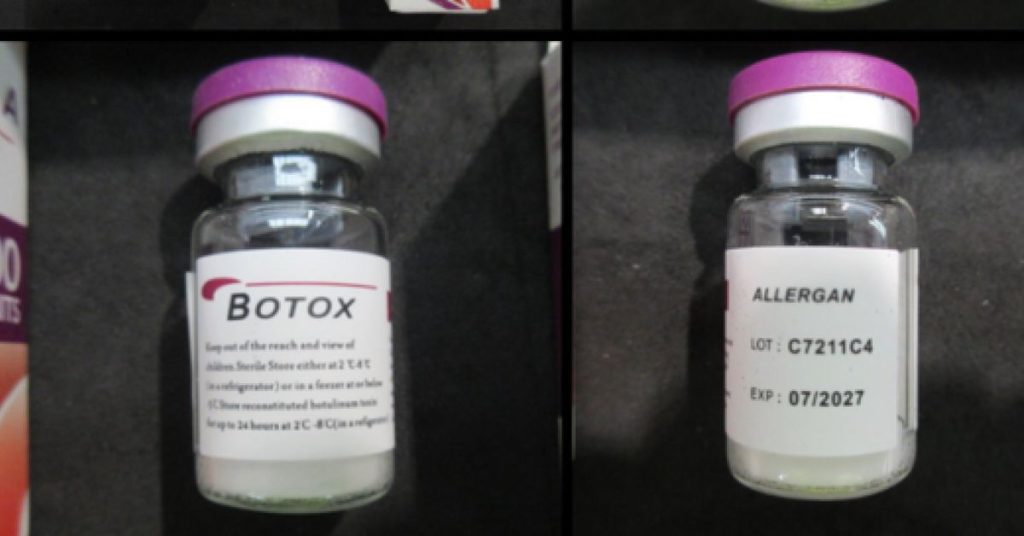
In recent weeks, multiple vials of fake Botox toxin have been stopped at border control, sparking concerns about the authenticity of these products imported from overseas. The Therapeutic Goods Administration (TGA) has offered clarification on this issue, suggesting that the boxes labeled as “off-the-shelf injectable Botox” appear genuine. These products are claimed to be假冒 Botox products produced by the company Allergan, which operates under the AB/BV brand, a prominent global pharmaceutical company.
However, despite their appearance, these boxes were later confirmed to contain inconsistencies in packaging. Notably, typographic errors, incorrect spacing, bolding, and spelling mistakes were noted. These issues were shared with AbbVie, the company producing the boxes, which later confirmed this information. The TGA has verified that the batch numbers on the boxes, C7211C4 and HA 33946, do not correspond to actual batches produced by AbbVie, yet these batches were obtained from an overseas supplier.
The key concern is the potential health risks associated with these Botox products. The TGA emphasized that such products can pose serious safety risks, particularly affecting Botox and simulatous diets. Using these products is not only concerning but also=q, as genuine products are regulated and have not been assessed for quality, safety, or efficacy.
Consumers are advised not to act, nor to acquire, these Botox products if they are suspicious about their origin. The TGA has explicitly stated that these products are not prescription-only, highlighting that counterfeit products are not considered to have been evaluated for standard safety. While Botox is generally a prescription-only substance in Australia, the health risks associated with its use must be avoided despite the claim that these products do not meet the same standards as other drugs.
Furthermore, counterfeits of Botox are frequently reported, and the TGA has noted that some individuals may be concerned that products obtained over the internet are counterfeit. To ensure safety and effectiveness, consumers should report any suspect products by contacting a healthcare professional or a trusted pharmacy.
In summary, the issue concerning fake Botox products highlight the need for caution with imports from overseas. The TGA’s clear explanation of inconsistencies and proposed guidelines emphasize the importance of evaluating the safety and efficacy of any healthcare product before compromising its use.